Expression of nano-antibody in different systems
? Foreword
In 1993, Belgian scientists discovered a new antibody-nano-antibody (VHHs) in camel serum, which is different from the traditional antibody structure and only consists of heavy chain variable regions. Compared with traditional antibodies, nano-antibodies have the advantages of stable physical and chemical properties, low immunogenicity, strong tissue permeability, and can recognize epitopes that traditional antibodies cannot recognize, which makes them a strong and beneficial supplement to traditional antibodies.
Nano-antibody is only composed of heavy chain variable region, with small molecular weight and simple structure, which enables it to realize stable expression in various expression systems. So far, VHHs has been expressed in many expression systems, from prokaryotic cells, yeast, fungi, insect cells, mammalian cell lines to plants. This paper mainly introduces the expression of nano-antibody in different expression systems, and the present situation and development trend in the field of this system.
Prokaryotic expression system Prokaryotic expression system is the earliest mature protein expression system studied. Obtaining foreign proteins through prokaryotes can obtain gene expression products in a short time, and the cost required is relatively low. The expression system mainly includes Escherichia coli expression, Bacillus subtilis expression and Streptomyces expression. Among them, Escherichia coli system is the most widely used, and the prokaryotic system generally refers to the expression of Escherichia coli.
Escherichia coli expression system Escherichia coli expression system is the most important expression platform of recombinant protein in laboratory environment, and the expression is controlled by lactose operon. The host of Escherichia coli system has the advantages of fast growth, simple culture, low price, clear genetic background and high protein expression. However, it can not modify the expression product after translation, and the expression product contains a lot of endotoxin and is easy to form inclusion bodies.
Common host bacteria of Escherichia coli expression system
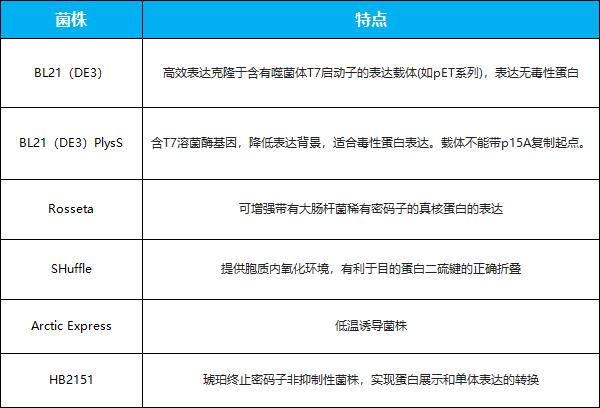
Periplasmic space, as the only compartment with oxidation environment in Escherichia coli, is the most commonly used expression system of nano-antibodies containing specific disulfide bonds. In addition to aerobic environment, there are many partners and isomerases in the periplasmic space, which can promote the correct formation of disulfide bonds and help protein fold. Nano-antibody protein is first produced in the cytoplasmic space, and then secreted into the periplasmic space through Sec pathway, SPR pathway or Tat pathway. After signal peptide is removed by signal peptidase, the target protein can be obtained [1].
Up to now, many VHHs have been successfully expressed in periplasm, some of which can reach tens of milligrams per liter, but there are still a lot of VHHs with low yield. This is because many fusion proteins can not be effectively secreted in the periplasmic space, and cytoplasmic expression seems to be the only expression mode of such fusion proteins [8].
The cytoplasm is a reduced compartment, so that the proteins expressed through the cytoplasm can not be folded correctly, and most of them are inclusion body proteins. The proteins are protected from the degradation of intracellular enzymes and are easy to be separated in high purity and concentration. However, there are many kinds of proteins in cytoplasm, and it is difficult to purify them. At the same time, nonfunctional inclusion proteins need to be completely denatured and refolded to be recovered from these inclusions, but the recovery of VHHs is usually inefficient [1]. In order to solve the problem of cytoplasmic expression, Xue X and others designed a new dialysis-dilution device in 2016. Using this device, researchers refolded VHHs and achieved a recovery rate of 85%, while the conventional dialysis and dilution methods only reached 13%[9]. Bacterial cytoplasm can be an effective compartment to produce correctly folded nanoparticles when expressed by recombinant vectors with sulfhydryl oxidase and disulfide isomerase activities. Larger volume and wider cytoplasm of chaperone/folding enzyme can achieve high yield of nano-antibody [6]. At the same time, double mutants with defects in thioredoxin and glutathione pathways have been developed. Some of these strains have been sold commercially in recent years, such as Origami (Novagen), Rosetta-gami (Novagen) and SHuffle?T7 (New England Biolabs) [10]. Among them, Shufflet 7 overexpresses a critical periplasmic isomerase DsbC, which can effectively catalyze the isomerization of disulfide bonds. Some studies have found that,The activity of isomerase DsbC can increase the yield of functional recombinant protein in periplasmic research [11].
Kavousipour Soudabeh and other modified vectors added four different signal peptide sequences to guide the secretion and expression of nano-antibodies, and found that TrBC signal peptide had the best effect [5]. Jianli Yu et al. used repetitive amino acid sequence motifs (GAGAGS) found in silk fibroin (SFP) as a new fusion tag (SF-tag) to enhance the expression of nanosomes in E.coli.. The transcription level of labeled protein is 2.3-7.0 times that of unlabeled protein, and the yield is increased by 2.5-7.1 times [27]. Xi Xie and others tried to select antifreeze protein AXX, and obtained its reverse transcription protein XXA by synthesizing XXA gene, which was used to develop new soluble fusion tags. It was found that the solubility ratio of fusion protein expressed by XXA fusion tag reached 86%, which was much higher than that of other tags [12].
Other prokaryotic expression systems Lactic acid bacteria are the most common intestinal probiotics, which are generally considered to be harmless to human body and are very suitable for the production host of therapeutic molecules. However, the existing expression data show that the yield of VHHS produced by lactic acid bacteria is low, about 1-3mg/L, which is much lower than that of Escherichia coli [13,14]. Lactobacillus rhamnosus DSM14870, as a normal flora in human vagina, can express HIV antibody in the form of soluble or cell wall anchor fixation, and is a suitable expression host for HIV antibody [2]. When Bacillus brevis choshinensis was used for 3L fed-batch fermentation, the yield was as high as 3g/L[15]. This system is easy to manipulate genes, has no endotoxin and shows weak protease activity, so it is a very noteworthy system.
Eukaryotic expression system
Due to the defects of prokaryotic expression system, such as difficult purification of inclusion body protein and incomplete protein modification, people began to use eukaryotic expression system to study genes. At present, eukaryotic expression systems commonly used in genetic engineering research include yeast expression system, insect cell expression system and mammalian cell expression system.
Yeast expression system Yeast system has clear genetic background, simple operation, easy genetic operation, perfect eukaryotic protein expression control system and advantages of both prokaryotic and eukaryotic expression systems. Yeast eukaryotic protein expression systems include methanol yeast expression system, Saccharomyces cerevisiae expression system, Schizosaccharomyces expression system and Kluyveromyces expression system [17].
Saccharomyces cerevisiae is the first yeast fermented by human beings, which has no specific virus and low cost. At present, it is the largest yeast expression system in shake flask culture, with the output exceeding 100mg/L[16]. Some amino acids in VHHs can participate in the recruitment of molecular chaperones in endoplasmic reticulum, thus narrowing the difference of molecular chaperones between Saccharomyces cerevisiae and mammalian cells, which is beneficial to the folding kinetics of nano-antibodies and improving the yield of nano-antibodies in Saccharomyces cerevisiae [17]. The protein expressed by Saccharomyces cerevisiae is prone to hyperglycosylation, and a large number of endogenous proteins are produced during fermentation, so the secretion efficiency of the target protein is low [1]. Considering the above problems, Saccharomyces cerevisiae is gradually replaced by methanol yeast represented by Pichia pastoris.
Pichia pastoris expression system is an efficient expression system of foreign proteins. This microorganism does not accumulate toxic ethanol, but only secretes a few endogenous proteins, and its glycosylation degree is the same as that of mammalian cells, so its immunogenicity is weak, so it is the most commonly used eukaryotic expression system. Pichia pastoris has a strictly regulated methanol inducible AOX1 promoter, which is suitable for high expression of foreign proteins [1]. But methanol is a highly toxic and dangerous chemical product. The GAP promoter without methanol induction is used instead of AXOI, and the carbon source does not need to be changed during the culture process, so the operation is simpler and the time for foreign proteins to reach the peak level can be shortened. The yield and affinity of nanoparticles produced by this system are similar to those produced by methanol-induced expression. It has been studied that the yield of nano-antibody expressed by this system in shake flask is 51.71mg/L[18] and 16mg/L[19].
Fungal expression system
Some filamentous fungi, such as Trichoderma and Aspergillus, can secrete a large number of foreign proteins in the culture medium. However, the fungal system not only expresses protein of interest, but also produces many proteases, which affects the yield. Joosten et al. obtained only 1.5 mg/L VHHs in Aspergillus awamori medium. After adding 0.25% BSA, it reached 7 mg/L. It is reported that more than 80% of VHH is degraded or adhered to the cell wall compared with endogenous xylanase secreted efficiently in this system, and researchers believe that the low secretion efficiency of VHHs is the bottleneck of fungal expression [20]. In order to overcome this limitation and protect VHHs from degradation, other researchers fused VHH with the high amylase A signal sequence (sTAA) of Rhizopus oryzae lipase (N28) and the N-terminal 28 amino acid fragment (N28), so that the highest yield of VHHs expressed in Aspergillus oryzae reached 73mg/L, which was 300-800 mg/L lower than that of Escherichia coli [21]. The final yield of GlaB-VHH fusion protein in Aspergillus oryzae can reach 610mg/L, which provides the possibility for high expression of nano-antibody, but its stability needs to be verified [22]. In addition, VHH[23] was expressed by maize head smut in unconventional secretion pathway.
Insect cell expression system
Insect cells are a widely used eukaryotic expression system, and recombinant baculovirus vectors (such as AcMNPV) can specifically transfect insect cells. Its survival in nature is short, and the strong promoter is inactivated in mammals and cannot infect vertebrates, so the recombinant baculovirus is safe for human beings. Proteins with complex structures under the control of strong promoters may not be able to form correct folding and post-translation modification, which may lead to the aggregation of target proteins and reduce antibody production, so the expression yield of VHHs in this system is much higher than that of other types of antibodies [17].
Gómez-Sebastián and others used the improved baculovirus expression system (IBES? technology) to express VHHs for the first time. The chigger larvae were inoculated with different recombinant baculovirus inoculation methods. The yield of VHH extracted from larvae is 257 mg/L, and the recovery rate after purification is 50%[7]. When expressing anti-endothelial cell selection protein, Drosophila cell SC-2 secreted 12-14mg/L, but the target protein was not detected when expressed in Escherichia coli at the same time, which may be related to the processing and modification ability of insect cells [26].
Mammalian cell expression system Mammalian cells are the best expression system for therapeutic antibodies, because the produced antibodies have the weakest immunogenicity. At present, the main cell lines include CHO, NS0, Sp2/0, HEK293 and per C6 [17]. Among them, CHO cells grow rapidly and adapt to serum-free suspension culture, which is the most popular host. They can express VHHS up to 100mg/L in shake flask [24]. HEK293 cells are more commonly used in the laboratory because of transient transfection and semi-stable expression, because they require less time and labor. However, monoclonal antibodies approved for the treatment of human diseases are only produced by CHO, NS0 and SP2/0. So far, HEK293 cells have not been used to produce VHHs, and there are few reports about producing antibody fragments by this method [17]. In most literatures, CHO is used to produce antibody fragments. Up to now, 50% of the therapeutic proteins produced in industry are still produced by CHO [1]. The production costs associated with mammalian cell lines are much higher than those associated with any other laboratory research platform.
Common host cells of mammalian expression system

Plant expression system Compared with other expression systems, plant production platform (stable and transient expression system) is cheap, safe, capable of folding and assembling complex proteins and post-translation modification, easy to expand, and unaffected by human pathogens and diseases. It is an ideal protein expression host and is usually used as an alternative method for mammalian expression systems. In addition, plant expression products can be concentrated in specific organs, such as leaves or seeds, and can be taken orally directly. Tobacco leaves are the most common hosts for expressing VHHs, while the proteins expressed in Arabidopsis and rice seeds have attracted much attention because of their direct oral input of VHHs[1]. The stable transformation methods commonly used in plant system expression have some problems, such as the recombinant gene escaping to nature or the time-consuming transformation. Therefore, some studies have proposed the transient expression system of plant virus expression vector as an alternative method, and the nano-body content with correct conformation reaches 0.45% of the total soluble protein [25].
Present situation and development of nano-antibody expression
Escherichia coli expression system is the most commonly used expression system of nano-antibody, which has two expression modes: periplasmic expression and cytoplasmic expression, each with its own advantages and disadvantages. Some oxidation engineering strains have been modified, but more experimental support is needed. In addition, various methods of modifying vectors, such as adding labels or signal peptides, have been put forward and have been verified by experiments. Compared with Escherichia coli, Pichia pastoris has excessive glycosylation, which may lead to immunogenicity and ultimately affect its clinical application. The expression systems of fungi, insects, plants and mammals are much more complicated than prokaryotic and eukaryotic expression systems, and are generally not easily adopted after considering the production time, cost and laboratory conditions.
Escherichia coli system is fast, convenient and cheap, and it is the first choice for VHH expression under most laboratory conditions. When some types of VHHs can’t be successfully produced in E.coli, other systems will be used instead.
List of nano-antibody expression
